Mathematics, 01.11.2019 08:31 oliviavaughan01
The ideal gas law states that the volume, v, of a gas in liters varies directly with the amount of the gas in moles, n, and the absolute temperature in kelvin, t, and varies inversely with the pressure, p, of the gas. two moles of a gas has a volume of 35.424 l at 270 kelvin when p = 1.25 atm. what is the volume of 3 moles of a gas at 280 kelvin when p = 1.5 atm?
a.11.48 l
b.27.33 l
c.45.92 l
d.103.32 l

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 13:30
Suppose the first five terms of a sequence are 4, 5, 9, 27, 123. how could the next term in the sequence be generated?
Answers: 1

Mathematics, 21.06.2019 17:00
The size of a certain cell is 2.5*10^-9m. another cell is 1.5*10^3 times larger. how large is the larger cell in scientific notation?
Answers: 2

Mathematics, 22.06.2019 00:00
Idon't get undoing if its pass adding and subtracting so can someone ? x-2 over 5 = 18
Answers: 1

Mathematics, 22.06.2019 00:30
Given abc find the values of x and y. in your final answer, include all of your calculations.
Answers: 1
You know the right answer?
The ideal gas law states that the volume, v, of a gas in liters varies directly with the amount of t...
Questions


Health, 20.06.2020 13:57


Mathematics, 20.06.2020 13:57

Biology, 20.06.2020 13:57


Mathematics, 20.06.2020 13:57








Mathematics, 20.06.2020 13:57

History, 20.06.2020 13:57




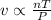
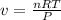 Equation 1
Equation 1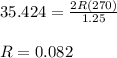
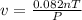 Equation 2
Equation 2