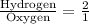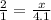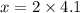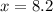Mathematics, 01.07.2019 02:30 titalili0204
The ratio of hydrogen to oxygen atoms in water molecules is 2 to 1. if the beaker of water contained 4.1 moles of oxygen atoms, how many moles of hydrogen atoms did it contain? (note: a mole is a unit commonly used in chemistry. you don't need to know this to solve the problem, but in case you are interested, 1 mole=6.02 times 10^23 atoms) plz !

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 16:50
Iq scores for adults age 20 to 34 years are normally distributed according to n(120, 20). in what range does the middle 68% of people in this group score on the test?
Answers: 1

Mathematics, 21.06.2019 20:00
Evaluate the discriminant of each equation. tell how many solutions each equation has and whether the solutions are real or imaginary. x^2 + 4x + 5 = 0
Answers: 2

Mathematics, 21.06.2019 20:00
1: 4 if the wew 35 surfboards at the beach how many were short boards?
Answers: 1

Mathematics, 21.06.2019 20:10
The constitution gives congress the power to create federal courts lower than the supreme court higher than the supreme court equal to the supreme court, unaffected by the supreme court.
Answers: 1
You know the right answer?
The ratio of hydrogen to oxygen atoms in water molecules is 2 to 1. if the beaker of water contained...
Questions




Mathematics, 24.08.2019 08:30





Health, 24.08.2019 08:30



Mathematics, 24.08.2019 08:30

Mathematics, 24.08.2019 08:30





Mathematics, 24.08.2019 08:30

Social Studies, 24.08.2019 08:30