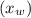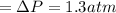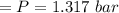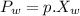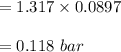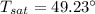n-Octane (C8H18) is burned with 60 percent excess air with 15 percent of the carbon in the fuel forming carbon monoxide. Calculate the mole fractions of the products and the dew-point temperature of the water vapor in the products when the products are at 1.3 atm pressure. Use data from the tables. The mole fraction of CO2 is .0678the mole fraction of CO is .012the mole fraction of H2O is .0897the mole fraction of O2 is .0808and the mole fraction of N2 is .7498The dew-point temperature of the water vapor is °C.

Answers: 3


Another question on English

English, 21.06.2019 17:10
Match each literary device to its definition. pun the use of humor and exaggeration to mock or criticize people's impractical thoughts and practices irony the use of words in a way that conveys the opposite of what they mean paradox the use of a word with more than one possible meaning with the intention of creating humor satire ideas or concepts that seem absurd or contradictory but are nevertheless true arrowboth arrowboth arrowboth arrowboth
Answers: 1

English, 21.06.2019 22:00
What is meant by “eternally burning black pipes”? what is this implying about the personality of the firemen?
Answers: 1

English, 22.06.2019 02:10
London includes a quote about john thornton as he is observing hal attempt to motivate the exhausted dogs "it was idle, he knew, to get between a fool and his folly". if the word "idle" is defined as "of no real worth, importance, or significance", what does this statement mean with regard to hal? who is the fool? what is hal's folly? why would john thornton think it of no real worth or useless to intervene?
Answers: 3

English, 22.06.2019 04:50
Whats the importance of gaining a general understanding of and interpretation of the work of literature. explain what the importance is to me
Answers: 3
You know the right answer?
n-Octane (C8H18) is burned with 60 percent excess air with 15 percent of the carbon in the fuel form...
Questions

History, 05.12.2019 23:31




Biology, 05.12.2019 23:31







Biology, 05.12.2019 23:31


Law, 05.12.2019 23:31


Chemistry, 05.12.2019 23:31




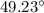 "
"