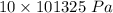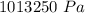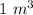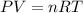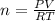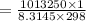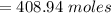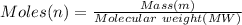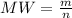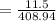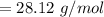Engineering, 28.06.2021 14:00 oliviaclerk5
In order to fill a tank of 1000 liter volume to a pressure of 10 atm at 298K, an 11.5Kg of the gas is required. How many moles of the gas are present in the tank? What is the molecular weight of the gas? Assuming that the gas to be a pure element can you identify it?

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Which of the following ziegler nichols tuning methods the response of the controller to a step input should exhibit an s-shaped curve? a)-open loop mode b)-closed loop mode c)-both modes (open & closed) d)-none of the modes (open & closed)
Answers: 3

Engineering, 04.07.2019 18:20
Inspection for bearing condition will include: (clo4) a)-color b)-smell c)-size d)-none of the above
Answers: 1

Engineering, 04.07.2019 18:20
Athin walled concentric tube exchanger is used to cool engine oil from 160°c to 60°c with water that is available at 25°c acting as a coolant. the oil and water flow rates are each at 2 kg/s, and the diameter of the inner tube is 0.5 m and the corresponding value of the overall heat transfer coefficient is 250 w/m2. oc. how long must the heat exchanger be to accomplish the desired cooling? cpwater=4.187 kj/kg-candcpengine el=2.035 kj/kg·°c, oil . 120]
Answers: 1

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3
You know the right answer?
In order to fill a tank of 1000 liter volume to a pressure of 10 atm at 298K, an 11.5Kg of the gas i...
Questions

Biology, 25.01.2022 02:00

Mathematics, 25.01.2022 02:00



Physics, 25.01.2022 02:00


Business, 25.01.2022 02:00

Mathematics, 25.01.2022 02:00

Mathematics, 25.01.2022 02:00



Mathematics, 25.01.2022 02:00



English, 25.01.2022 02:00


Mathematics, 25.01.2022 02:00


History, 25.01.2022 02:00