
Engineering, 17.12.2020 17:40 Carriepruitt
An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has the same value at the beginning and end. If the volume changes from Vi to Vf , the change in entropy is given by:

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of m 1.5 kg of steam is contained in a closed rigid container. initially the pressure and temperature of the steam are: p 1.5 mpa and t 240°c (superheated state), respectively. then the temperature drops to t2= 100°c as the result of heat transfer to the surroundings. determine: a) quality of the steam at the end of the process, b) heat transfer with the surroundings. for: p1.5 mpa and t 240°c: enthalpy of superheated vapour is 2900 kj/kg, specific volume of superheated vapour is 0. 1483 m/kg, while for t 100°c: enthalpy of saturated liquid water is 419kj/kg, specific volume of saturated liquid water is 0.001043m/kg, enthalpy of saturated vapour is 2676 kj/kg, specific volume of saturated vapour is 1.672 m/kg and pressure is 0.1 mpa.
Answers: 3

Engineering, 03.07.2019 14:10
If the thermal strain developed in polyimide film during deposition is given as 0.0044. assume room temperature is kept at 17.3 c, and thermal coefficient of expansion for the film and the substrate are 54 x 10^-6c^-1 and 3.3 x 10^-6c^-1respectively. calculate the deposition temperature.
Answers: 3

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:10
Awall of 0.5m thickness is to be constructed from a material which has average thermal conductivity of 1.4 w/mk. the wall is to be insulated with a material having an average thermal conductivity of 0.35 w/mk so that heat loss per square meter shall not exceed 1450 w. assume inner wall surface temperature of 1200°c and outer surface temperature of the insulation to be 15°c. calculate the thickness of insulation required.
Answers: 3
You know the right answer?
An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has...
Questions

English, 22.06.2019 03:00

Social Studies, 22.06.2019 03:00

Mathematics, 22.06.2019 03:00


Mathematics, 22.06.2019 03:00



English, 22.06.2019 03:00

History, 22.06.2019 03:00



History, 22.06.2019 03:00


Social Studies, 22.06.2019 03:00


Mathematics, 22.06.2019 03:00

Social Studies, 22.06.2019 03:00



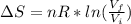
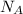 k, k is Boltzmann's constant in J K⁻¹ and Avogadro's constant
k, k is Boltzmann's constant in J K⁻¹ and Avogadro's constant 

