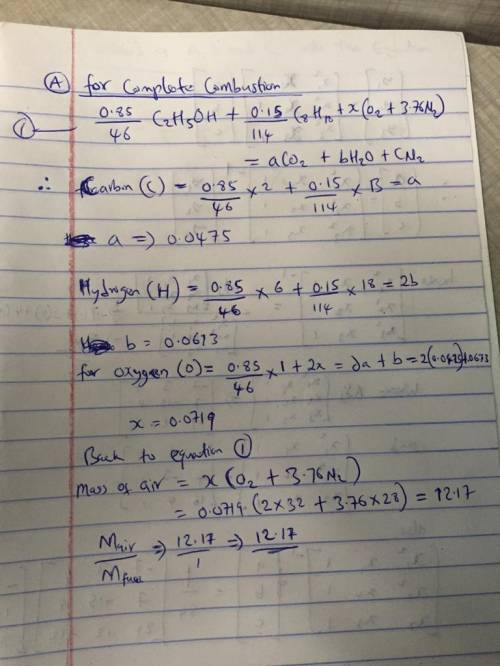Engineering, 29.11.2020 22:50 xeno777
An automotive fuel has a molar composition of 85% ethanol (C2H5OH) and 15% octane (C8H18). For complete combustion in air, determine: (a) the molar air-fuel ratio and air-fuel ratio by mass. (b) the lower heating value, in kJ per kmol of fuel and in kJ per kg of fuel.(c) the higher heating value, in kJ per kmol of fuel and in kJ per kg of fuel. (d) the dew point temperature of the combustion products at 1 atm, in °C. Consider a reference temperature and pressure of 25°C, 1 atm.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
Line joining liquid phase with liquid and solid phase mixture is known as: a) liquidus b) solidus c) tie line d) none of the mentioned
Answers: 2

Engineering, 04.07.2019 18:20
Describe one experiment in which the glass transition temperature and melting temperature of a totally amorphous thermoplastic material can be determined. show the relevant experimental results in a diagram which should be properly annotated with the two temperatures clearly marked. what is likely to happen to the curve in the diagram if the amorphous polymer is replaced by a thermosetting type?
Answers: 2

Engineering, 04.07.2019 19:10
What are the major differences between injection molding and extrusion?
Answers: 2

Engineering, 04.07.2019 19:10
What is the chief metrological difference between measuring with a microscope and with an electronic comparator? a. the microscope is limited to small workpieces.a. the microscope is limited to small workpieces. c. the comparator can only examine one point on the workpiece. d. the microscope carries its own standard.
Answers: 1
You know the right answer?
An automotive fuel has a molar composition of 85% ethanol (C2H5OH) and 15% octane (C8H18). For compl...
Questions

Mathematics, 18.04.2021 01:00




English, 18.04.2021 01:10

Mathematics, 18.04.2021 01:10

Mathematics, 18.04.2021 01:10


English, 18.04.2021 01:10





Mathematics, 18.04.2021 01:10

Health, 18.04.2021 01:10


Mathematics, 18.04.2021 01:10

English, 18.04.2021 01:10