
Engineering, 16.10.2020 09:01 ally6440
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative predictions about these macroscopic properties by thinking on a microscopic scale. Kinetic theory and statistical mechanics provide a way to relate molecular models to thermodynamics. Predicting the heat capacities of gases at a constant volume from the number of degrees of freedom of a gas molecule is one example of the predictive power of molecular models. The molar specific heat Cv of a gas at a constant volume is the quantity of energy required to raise the temperature T of one mole of gas by one degree while the volume remains the same. Mathematically, Cv=1nΔEthΔT, where n is the number of moles of gas, ΔEth is the change in internal (or thermal) energy, and ΔT is the change in temperature. Kinetic theory tells us that the temperature of a gas is directly proportional to the total kinetic energy of the molecules in the gas. The equipartition theorem says that each degree of freedom of a molecule has an average energy equal to 12kBT, where kB is Boltzmann's constant 1.38×10^−23J/K. When summed over the entire gas, this gives 12nRT, where R=8.314Jmol⋅K is the ideal gas constant, for each molecular degree of freedom.
Required:
a. Using the equipartition theorem, determine the molar specific heat, Cv , of a gas in which each molecule has s degrees of freedom. Express your answer in terms of R and s.
b. Given the molar specific heat Cv of a gas at constant volume, you can determine the number of degrees of freedom s that are energetically accessible. For example, at room temperature cis-2-butene, C4H8 , has molar specific heat Cv=70.6Jmol⋅K . How many degrees of freedom of cis-2-butene are energetically accessible?

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of m 1.5 kg of steam is contained in a closed rigid container. initially the pressure and temperature of the steam are: p 1.5 mpa and t 240°c (superheated state), respectively. then the temperature drops to t2= 100°c as the result of heat transfer to the surroundings. determine: a) quality of the steam at the end of the process, b) heat transfer with the surroundings. for: p1.5 mpa and t 240°c: enthalpy of superheated vapour is 2900 kj/kg, specific volume of superheated vapour is 0. 1483 m/kg, while for t 100°c: enthalpy of saturated liquid water is 419kj/kg, specific volume of saturated liquid water is 0.001043m/kg, enthalpy of saturated vapour is 2676 kj/kg, specific volume of saturated vapour is 1.672 m/kg and pressure is 0.1 mpa.
Answers: 3

Engineering, 03.07.2019 23:20
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10
Apipe with an outside diameter of 15 cm is exposed to an ambient air and surrounding temperature of -20°c. the pipe has an outer surface temperature of 65°c and an emissivity of 0.85. if the rate of heat loss from the pipe surface is 0.95 kw per meter of length, the external convective heat transfer coefficient (h) is: (a) 12.5 w/m"k (b) 18.6 w/mk (c) 23.7 w/mk (d) 27.9 w/mk (e) 33.5 w/mk
Answers: 1

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2
You know the right answer?
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative...
Questions


English, 16.08.2019 09:10

Arts, 16.08.2019 09:10



Chemistry, 16.08.2019 09:10

History, 16.08.2019 09:10





Mathematics, 16.08.2019 09:10


Mathematics, 16.08.2019 09:10

History, 16.08.2019 09:10



Mathematics, 16.08.2019 09:10

Mathematics, 16.08.2019 09:10

Social Studies, 16.08.2019 09:10

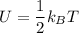
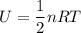
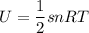
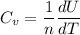
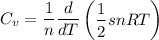
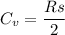
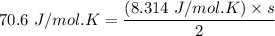
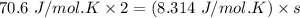
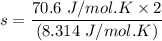
 17
17

