Engineering, 12.10.2020 15:01 BreBreDoeCCx
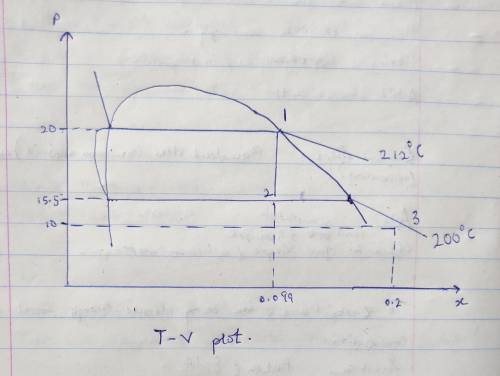
Steam at 20 bars is in the saturated vapor state (call this state 1) and contained in a pistoncylinderdevice with a volume of 0.03 m3. Assume the steam is cooled at constantvolume (i. e. the piston is held fixed in place) until the temperature reaches 200 C (callthis state 2). Then the steam is expanded isothermally until its volume is three times theinitial value (state 3).
Required:
a. Determine the pressures at state 2 and 3. ans. 15.5 bar, ~10 bar
b. Determine the change in specific internal energy, u, for each of the two processes.
-389 kJ/kg, 410 kJ/kg
c. Make qualitatively correct sketches of the processes on a T-v plot.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:10
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:10
Which one from below is not one of the reasons of planning failures? (clo3) a)-planner is careless. b-planner spend less time in the field but more time on the desk c)-planner is not qualified d)-planner does not have sufficient time to properly plan
Answers: 3

Engineering, 04.07.2019 18:20
Apiston-cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kpa. heat is transferred at constant pressure until the temperature of water reaches 350 °c. determine (a) the quality of water at the initial state (b) the work associated with this process, (c) the heat associated with this process.
Answers: 2
You know the right answer?
Steam at 20 bars is in the saturated vapor state (call this state 1) and contained in a pistoncylind...
Questions


English, 18.11.2020 01:00

Advanced Placement (AP), 18.11.2020 01:00


Mathematics, 18.11.2020 01:00


History, 18.11.2020 01:00

English, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

History, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Physics, 18.11.2020 01:00

Social Studies, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Physics, 18.11.2020 01:00

English, 18.11.2020 01:00



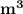
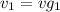 = 0.0996
= 0.0996 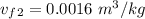
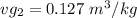
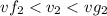 , the saturated pressure at state 2 i.e. P₂ = 15.5 bar
, the saturated pressure at state 2 i.e. P₂ = 15.5 bar 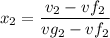
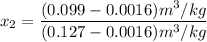
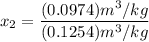
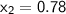
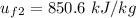 , also
, also 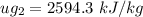
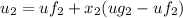
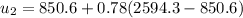
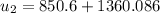
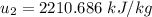
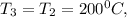
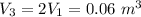
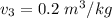
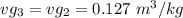 ,
,  , therefore, the phase is in a superheated vapour state.
, therefore, the phase is in a superheated vapour state.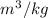
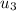 at the pressure of 10 bar = 2622.3 kJ/kg
at the pressure of 10 bar = 2622.3 kJ/kg