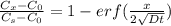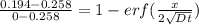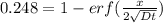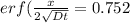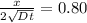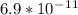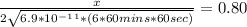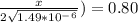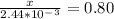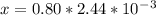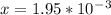Engineering, 18.06.2020 15:57 zara76
An iron-carbon alloy initially containing 0.258 wt% C is exposed to an oxygen-rich and virtually carbon-free atmosphere at 1120°C. Under these circumstances the carbon diffuses from the alloy and reacts at the surface with the oxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0.0 wt% C. At what position will the carbon concentration be 0.194 wt% after a 6 h treatment? The value of D at 1120°C is 6.9 × 10-11 m2/s.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 03.07.2019 19:30
When using the ohmmeter function of a digital multimeter, the leads are placed in what position relative to the component being tested? a. parallel b. control c. series d. line
Answers: 3

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3
You know the right answer?
An iron-carbon alloy initially containing 0.258 wt% C is exposed to an oxygen-rich and virtually car...
Questions



History, 28.07.2019 18:30

English, 28.07.2019 18:30

Health, 28.07.2019 18:30

Biology, 28.07.2019 18:30


Biology, 28.07.2019 18:30

Mathematics, 28.07.2019 18:30





Mathematics, 28.07.2019 18:30

English, 28.07.2019 18:30

Mathematics, 28.07.2019 18:30




Social Studies, 28.07.2019 18:30