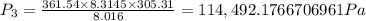Engineering, 22.04.2020 23:11 Serenaark2834
An insulated rigid tank is divided into two compartments by a partition. One compartment contains 7 kg of oxygen gas at 40°C and 100 kPa, and the other compartment contains 4 kg of nitrogen gas at 20°C and 150 kPa. Now the partition is removed, and the two gases are allowed to mix. Determine
(a) the mixture temperature and
(b) the mixture pressure after equilibrium has been established.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 19:30
When using the ohmmeter function of a digital multimeter, the leads are placed in what position relative to the component being tested? a. parallel b. control c. series d. line
Answers: 3

Engineering, 04.07.2019 18:10
Which of the following controllers anticipates the future from the slope of errors over time? a)-proportional b)-on/off c)-integral d)-derivative.
Answers: 2

Engineering, 04.07.2019 18:20
For a gate width of 2 m into the paper, determine the force required to hold the gate abc at its location.
Answers: 1

Engineering, 04.07.2019 19:20
At steady state, air at 200 kpa, 325 k, and mass flow rate of 0.5 kg/s enters an insulated duct having differing inlet and exit cross-sectional areas. the inlet cross-sectional area is 6 cm2. at the duct exit, the pressure of the air is 100 kpa and the velocity is 250 m/s. neglecting potential energy effects and modeling air as an 1.008 kj/kg k, determine ideal gas with constant cp = (a) the velocity of the air at the inlet, in m/s. (b) the temperature of the air at the exit, in k. (c) the exit cross-sectional area, in cm2
Answers: 2
You know the right answer?
An insulated rigid tank is divided into two compartments by a partition. One compartment contains 7...
Questions

Mathematics, 27.01.2020 02:31

English, 27.01.2020 02:31

History, 27.01.2020 02:31


Biology, 27.01.2020 02:31



Mathematics, 27.01.2020 02:31

Social Studies, 27.01.2020 02:31



Chemistry, 27.01.2020 02:31

Mathematics, 27.01.2020 02:31


Mathematics, 27.01.2020 02:31

Biology, 27.01.2020 02:31


Social Studies, 27.01.2020 02:31

History, 27.01.2020 02:31

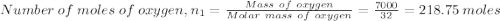
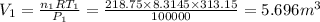
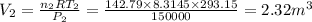
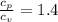
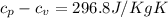
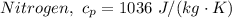
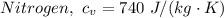
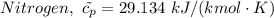
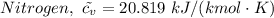
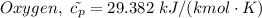
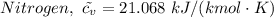
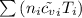 = 0.21875 × 21.068 × 313.15 + 0.14279×20.819×293.15 = 2,314.65 kJ
= 0.21875 × 21.068 × 313.15 + 0.14279×20.819×293.15 = 2,314.65 kJ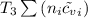 = T (0.21875 × 21.068 + 0.14279×20.819) = T×7.58137001
= T (0.21875 × 21.068 + 0.14279×20.819) = T×7.58137001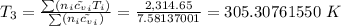
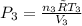
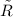 = 8.3145 J/(gmol·K)
= 8.3145 J/(gmol·K)