Engineering, 18.04.2020 02:00 tcjet
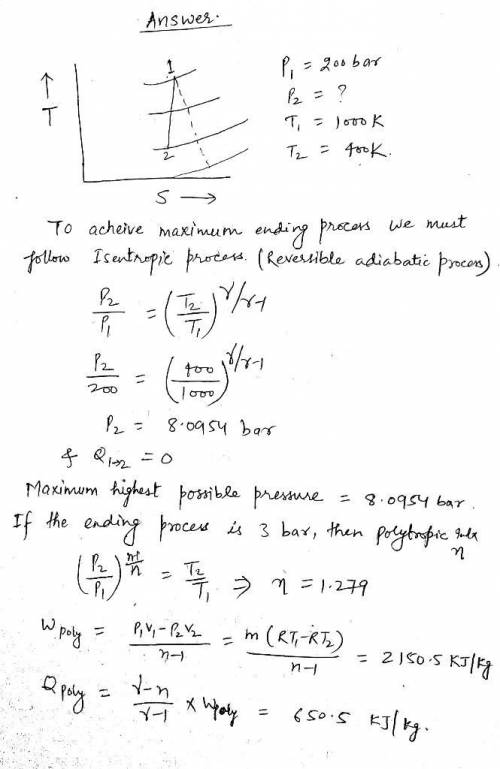
Air as an ideal gas in a closed system undergoes a reversible process between temperatures of 1000 K and 400 K. The beginning pressure is 200 bar. Determinc the highest possible ending pressure for this process. If the ending pressure is 3 bar, determine the heat transfer and work per unit mass, if the boundary of the system is in constant contact with a reservoir at 400 K.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1

Engineering, 04.07.2019 18:10
What are the two (02) benefits, which may result from a successful implementation of preventive maintenance (pm) program in an organization? (clo3)a)- lean manufacturing b)-overlapping responsibilities c)-the planner is not qualified d)-accurate contractor information e)-reduction in equipment redundancies f)-accurate stores information
Answers: 3
You know the right answer?
Air as an ideal gas in a closed system undergoes a reversible process between temperatures of 1000 K...
Questions

English, 08.04.2020 18:36



Mathematics, 08.04.2020 18:36




Arts, 08.04.2020 18:36

History, 08.04.2020 18:36


Mathematics, 08.04.2020 18:36

Business, 08.04.2020 18:36




Mathematics, 08.04.2020 18:36


Chemistry, 08.04.2020 18:36


English, 08.04.2020 18:37