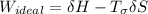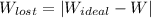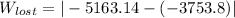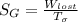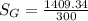Engineering, 11.04.2020 02:51 claudia3776
A steady flow adiabatic turbine accepts gas at conditions T1, P1 and discharges at conditions T2 and P2. Assuming ideal gas, determine (per mole of gas) W, Wideal, Wlost and SG for the following. Take Tσ = 300 Κ, Τ1 = 500 Κ, P1 = 6 bar, Τ2 = 371 Κ, P2 = 1.2 bar, and Cp/R = 7/2.
Chemical Engineering (thermodynamics) Please answer as soon as possible.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
If a particle moves along a path such that r : (3 sin t) m and ? : 2t rad, where t is in seconds. what is the particle's acceleration in m/s in 4 seconds? a)- 16.43 b)- 16.29 c)- 15.21 d)- 13.79
Answers: 1

Engineering, 04.07.2019 18:20
Steam enters a converging nozzle at 3.0 mpa and 500°c with a at 1.8 mpa. for a nozzle exit area of 32 cm2, determine the exit velocity, mass flow rate, and exit mach number if the nozzle: negligible velocity, and it exits (a) is isentropic (b) has an efficiency of 94 percent
Answers: 2


Engineering, 04.07.2019 19:10
Estimate the change in specific internal energy au and specific enthalpy h from inlet to outlet for ethylene glycol (a liquid) flowing through each of the following devices: (a) a heat exchanger where the glycol temperature increases from 20 °c to 80 °c; (b) a pump operating at about 25 °c and increasing the glycol pressure from 100 kpa to 8 mpa.
Answers: 2
You know the right answer?
A steady flow adiabatic turbine accepts gas at conditions T1, P1 and discharges at conditions T2 and...
Questions

Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

History, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


Physics, 09.01.2021 01:00



Health, 09.01.2021 01:00


Arts, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

History, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


History, 09.01.2021 01:00

History, 09.01.2021 01:00

English, 09.01.2021 01:00


Chemistry, 09.01.2021 01:00

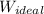 = - 5163.14 J
= - 5163.14 J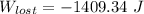
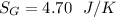
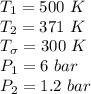
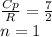
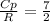
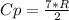
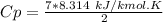
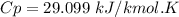
![n[Cp In \frac{T_2}{T_1} - R In \frac{P_2}{P_1}]](/tpl/images/0594/9077/c65c6.png)
![1*[29.099 In(\frac{371}{500}) - 8.314 In (\frac{1.2}{6})]](/tpl/images/0594/9077/c221b.png)