
Engineering, 08.04.2020 04:36 gorbyalexis
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pressure of 300 kPa. The gas is heated, and the gage pressure at the final state is 600 kPa. The local atmospheric pressure is 1 atm. Determine the final temperature, in °C.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Ariver flows from north to south at 8 km/h. a boat is to cross this river from west to east at a speed of 20 km/h (speed of the boat with respect to the earth/ground). at what angle (in degrees) must the boat be pointed upstream such that it will proceed directly across the river (hint: find the speed of the boat with respect to water/river)? a 288 b. 21.8 c. 326 d. 30.2
Answers: 3

Engineering, 04.07.2019 18:10
Burgers vector is generally parallel to the dislocation line. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3

Engineering, 04.07.2019 18:20
Atank with constant volume contains 2.27 kg of a mixture of water phases (liquid-vapor). in the initial state the temperature and the quality are 127 °c and 0.6, respectively. the mixture is heated until the temperature of 160 oc is reached. illustrate the process in a t-v diagram. then, determine (1) the mass of the vapor in kg at the initial state, (2) the final pressure in kpa.
Answers: 3
You know the right answer?
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pres...
Questions

Mathematics, 01.04.2021 18:20


Chemistry, 01.04.2021 18:20


Mathematics, 01.04.2021 18:20





Mathematics, 01.04.2021 18:20

Mathematics, 01.04.2021 18:20



Computers and Technology, 01.04.2021 18:20

Mathematics, 01.04.2021 18:20

Mathematics, 01.04.2021 18:20




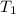 = 60 °C = 333.15 K Initial temperature
= 60 °C = 333.15 K Initial temperature 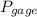 = 300 kPa , gage initial pressure ,
= 300 kPa , gage initial pressure ,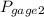 = 600 kPa , gage final pressure ,
= 600 kPa , gage final pressure ,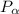 = 101.325 kPa
= 101.325 kPa 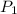 =
= 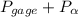
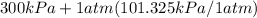
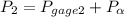
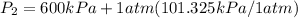
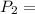 600. 299 kPa
600. 299 kPa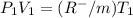 this is equation one
this is equation one 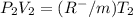 this is equation two
this is equation two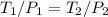
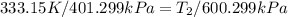
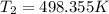 = 225.205 °C
= 225.205 °C

