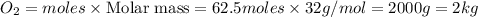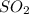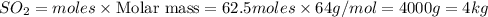Engineering, 07.04.2020 03:29 id0617045
Trace amounts of sulfur (S) in coal are burned in the presence of diatomic oxygen (O2) to form sulfur dioxide (SO2). Determine the minimum mass of oxygen required in the reactants and the mass of sulfur dioxide in the products when 2 kg of sulfur is burned.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:20
Select any two (2) areas of applications of chain-drive. (clo4) a)-permanent lubrication necessary b)-hydraulic forklift truck operation c)-rigging and heavy moving materials d)-relatively high maintenance costs e)-costlier than belt drives
Answers: 2

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3
You know the right answer?
Trace amounts of sulfur (S) in coal are burned in the presence of diatomic oxygen (O2) to form sulfu...
Questions


Health, 27.06.2019 13:30


Mathematics, 27.06.2019 13:30


History, 27.06.2019 13:30

English, 27.06.2019 13:30

History, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30

Social Studies, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30


Business, 27.06.2019 13:30

Mathematics, 27.06.2019 13:30



English, 27.06.2019 13:30

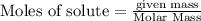
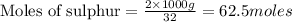
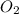
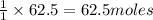 of
of