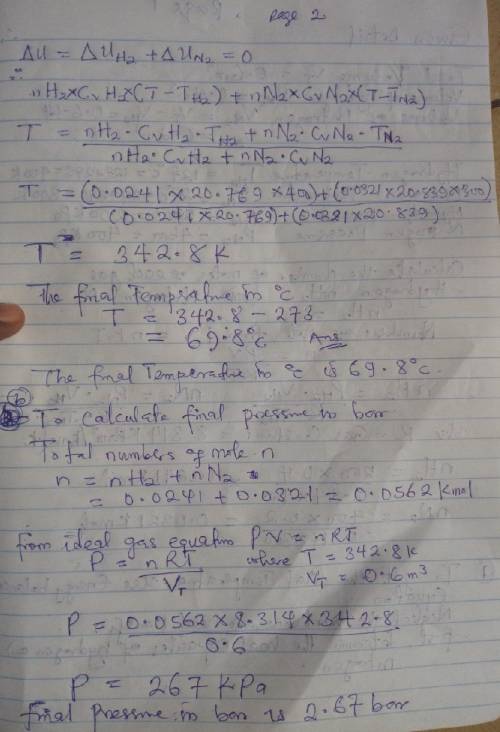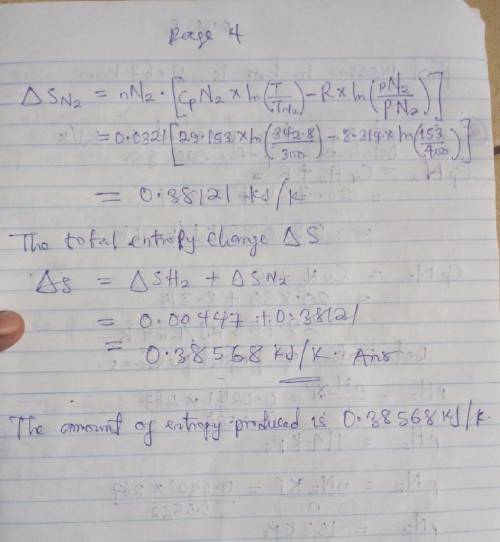Engineering, 30.03.2020 20:35 angel41vgg
An insulated tank having a total volume of 0.6 m3 is divided into two compartments. Initially one compartment contains 0.4 m3 of hydrogen at 127 oC, 2 bar and the other contains nitrogen at 27 oC, 4 bar. The gases are allowed to mix until an equilibrium state is attained. Assume the ideal gas model with constant specific heats, determine
a. The final temperature, in oC.
b. The final pressure, in bar.
c. The amount of entropy produced, in KJ/K.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 18:20
Determine the damped natural frequencies and the steady state response of a decoupled damped forced two degrees of freedom system. 10ä1 + 2q1 20q1 10 cos t; 10q2 +4q2 + 40q2 10 cos t
Answers: 3
You know the right answer?
An insulated tank having a total volume of 0.6 m3 is divided into two compartments. Initially one co...
Questions



Mathematics, 10.07.2019 03:30

Biology, 10.07.2019 03:30

Chemistry, 10.07.2019 03:30

Chemistry, 10.07.2019 03:30



English, 10.07.2019 03:30

Social Studies, 10.07.2019 03:30

History, 10.07.2019 03:30





Chemistry, 10.07.2019 03:30

Mathematics, 10.07.2019 03:30

Mathematics, 10.07.2019 03:30


Social Studies, 10.07.2019 03:30