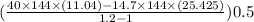Engineering, 17.03.2020 04:55 fluffyskunk302
A piston-cylinder assembly contains 0.5 lb of water. The water expands from an initial state where p1 = 40 lbf/in.2 and T1 = 300o F to a final state where p2 = 14.7 lbf/in.2 During the process, the pressure and specific volume are related by the polytropic process pv 1.2 = constant. Determine the energy transfer by work, in Btu.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 04.07.2019 18:10
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 19:10
Air inially occupying a volume of 1 m2 at 100 kpa, 27 c undergoes three internally reversible processes in series. process 1-2 compression to 500 kpa during which pv constant process 2-3 adiabatic expanslon to 100 kpa process 3-1: constant-pressure expansion to 100 kpa (a) calculate the change of entropy for each of the three processes. (b) calculate the heat and work involved in each process. (c) is this cycle a power cycle or refrigeration cycle?
Answers: 3
You know the right answer?
A piston-cylinder assembly contains 0.5 lb of water. The water expands from an initial state where p...
Questions

English, 21.08.2019 20:00




English, 21.08.2019 20:00


Biology, 21.08.2019 20:00


Mathematics, 21.08.2019 20:00


Mathematics, 21.08.2019 20:00


Mathematics, 21.08.2019 20:00

Mathematics, 21.08.2019 20:00



Mathematics, 21.08.2019 20:00

Social Studies, 21.08.2019 20:00

Mathematics, 21.08.2019 20:00

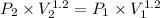 ,...............1
,...............1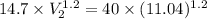
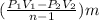 ...............2
...............2