Engineering, 11.03.2020 01:02 ashmarie69
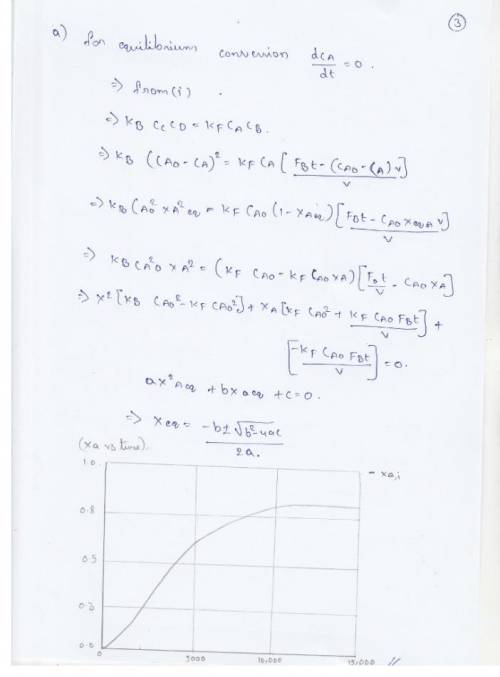
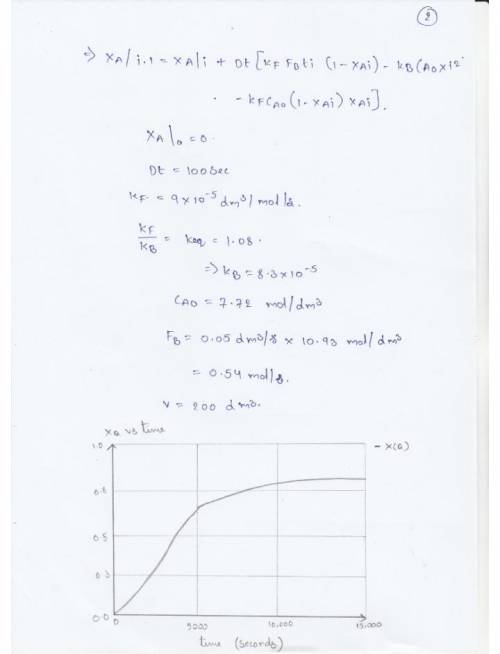
Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol. The reaction
CH_3COOC_2H_5 + C_4H_9OH <> CH_3COOC_4H_9 + C_2H_5OH
is elementary and reversible. The reaction is carried out isothermally at 300 K. At this the equilibrium constant is 1.08 and the specific reaction rate is 9 x 10^-5 dm^3/mol*s. Initially, there is 200 dm^3 of ethyl acetate in the vat, and butanol is fed at a volumetric rate of 0.05 dm^3/ s. The feed and initial concentrations of butanol and ethyl acetate are 10.93 mol/dm^3 and .72 molu/dm^3, respectively.
(a) Plot and analyze the equilibrium conversion of ethyl acetate as a function of time.
(b) Plot and analyze the conversion of ethyl acetate, the rate of reaction, and the concentration of butanol as a function of time.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
Ahot wire operates at a temperature of 200°c while the air temperature is 20°c. the hot wire element is a tungsten wire of 5 um diameter and 2 mm in length. plot using excel current, heat transfer and heat generated by the wire for air velocity varying from 1-10 m/s in steps of lm/s? matlab the sensor voltage output, resistance, or assume nu 0.989 re033pr13 take air properties at tr (200°c20°c)/2 = 110°c properties of tungsten: c 0.13 kj/kg.k 3 p 19250 kg/m k (thermal conductivity) = 174 w/m.k
Answers: 2

Engineering, 04.07.2019 18:10
Which one from below is not one of the reasons of planning failures? (clo3) a)-planner is careless. b-planner spend less time in the field but more time on the desk c)-planner is not qualified d)-planner does not have sufficient time to properly plan
Answers: 3

Engineering, 04.07.2019 18:20
Derive the correction factor formula for conical nozzle i=-(1+ cosa) and calculate the nozzle angle correction factor for a nozzle whose divergence hal-fangle is 13 (hint: assume that all the mass flow originates at the apex of the cone.
Answers: 3

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl ac...
Questions



Business, 01.02.2020 07:42

History, 01.02.2020 07:42

Mathematics, 01.02.2020 07:42

History, 01.02.2020 07:42

Biology, 01.02.2020 07:43




Social Studies, 01.02.2020 07:43

Business, 01.02.2020 07:43



Computers and Technology, 01.02.2020 07:43

Computers and Technology, 01.02.2020 07:43


English, 01.02.2020 07:43

Mathematics, 01.02.2020 07:43

Mathematics, 01.02.2020 07:43