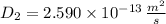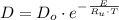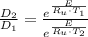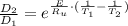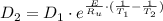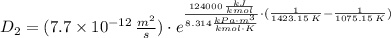Engineering, 07.03.2020 02:44 amber3687
The activation energy for the diffusion of atomic species A in metal B is 124 kJ/mol. Calculate the diffusion coefficient at 802°C, given that the value of D at 1150°C is 7.7 × 10-12 m2/s.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 19:10
For a process taking place in a closed system containing gas, the volume and pressure relationship is pvi-constant. -1.5 bar, the process starts with initial conditions, pi = =0.03 m3 and ends with final volume, v2-0.05 m3 determine the work done by the gas.
Answers: 2

Engineering, 04.07.2019 19:20
A5 kg block of fe is dropped into a very large vat of water. the fe and water initial temperatures are 95 and 25 c, respectively. the fe final temperature is 25 c and the water can be treated as a thermal reservoir,. treated as a thermal reservoir take the water to be the system and determine the entropy generation. report vour answer in kj/k.
Answers: 1

Engineering, 04.07.2019 19:20
Acompressor compresses a gas, a pump compresses a liquid. for a given pressure ratio, why does it take more work to compress a gas in a compressor than a liquid in a pump? a)- for a given pressure ratio the average specific volume for a gas is much higher than the average specific volume for a liquid. b)- there is no difference. the only difference is the amount of heat generated (not work) c)- for a given pressure ratio the average volurge for a gas is much higher than the average volume for a liquid. d)-there is no difference
Answers: 3
You know the right answer?
The activation energy for the diffusion of atomic species A in metal B is 124 kJ/mol. Calculate the...
Questions



Mathematics, 29.06.2019 11:00

Mathematics, 29.06.2019 11:00





Mathematics, 29.06.2019 11:00

Geography, 29.06.2019 11:00



English, 29.06.2019 11:00

Mathematics, 29.06.2019 11:00