The ideal gas equation states that:
P = nRT/V
where P is the pressure, V is the volume,...

Engineering, 06.03.2020 23:23 shayyy49
The ideal gas equation states that:
P = nRT/V
where P is the pressure, V is the volume, T is the temperature, R = 0.08206 (L atm) / (mol K) is the gas constant, and n is the number of moles.
Real gases, especially at high pressure, deviate from this behavior. Their response can be modeled with the van der
Waals equation:
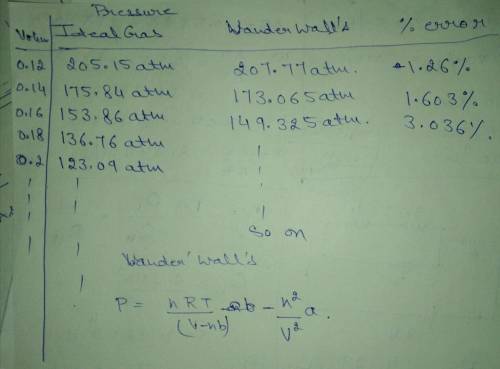
P = nRT/V−nb − n^2a/V^2
where a and b are material constants.
1. Consider 1 mole ( n = 1 ) of nitrogen gas at T = 300K. (For nitrogen gas a = 1.39 (L^2 atm)/mol^2, and b = 0.0391 L/mol.), create a vector with values of Vs for 0.1 <_ V<_1 L, using increments of 0.02 L.
2. Using this vector calculate P twice for each value of V, once using the ideal gas equation and once with the van der Waals equation. Using the two sets of values for P, calculate the percent of error
((P waal - Pwaals / Pwaals) 100) for each value of V.
3. Finally, by using MATLAB's built-in function max, determine the maximum error and the corresponding volume.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Coiled springs ought to be very strong and stiff. si3n4 is a strong, stiff material. would you select this material for a spring? explain.
Answers: 2

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:10
Shafts are machine elements that are used to a) carry axial loads b) direct shear loads c) transmit power d) rotate at constant speed e) none of the above circular and square shafts subjected to the same torque under the same circum behave a) the same way b) almost the same way
Answers: 2

Engineering, 04.07.2019 18:10
Draw the engineering stress-strain curve for (a) bcc; (b) fcc metals and mark important points.
Answers: 1
You know the right answer?
Questions




Computers and Technology, 22.06.2019 09:00





Mathematics, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

Biology, 22.06.2019 09:00





Biology, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00