Engineering, 27.02.2020 17:57 karyme12
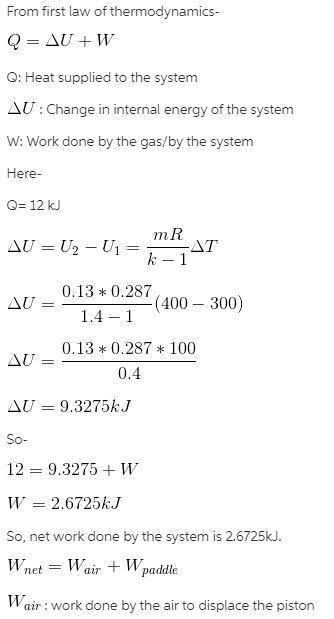
A piston cylinder assembly fitted with a slowly rotating paddle wheel contains 0.13 kg of air at 300K. The air undergoes a constant pressure process to a final temp of 400K. During the process heat is transfered to the air by Q=12kJ. Assuming the ideal gas model with k=1.4 and negligible changes in kinetic and potential energy for the air, determine the work done by the paddle on the air and the work done by the air to displace the piston. Gas constant for air is 0.287 kJ/kg*K
note ideal gas model is pV=mRT, and with k=1.4 the total internal energy change of air canbe calculated by U2-U1=(mR/k-1)(delta T)

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 18:10
Compute the pressure drop of 30°c air flowing with a mean velocity of 8 m/s in a circular sheet-metal duct 300 mm in diameter and 15 m long. use a friction factor, f 0.02, and pair = 1.1644 kg/m a. 37.26 pa b. 25.27 pa n c. 29.34 pa d. 30.52 pa
Answers: 1

Engineering, 04.07.2019 18:20
Aquick transition of the operating speed of a shaft from its critical speed will whirl amplitude. (a) increase (b) limit (c) not affect (d) zero
Answers: 2

Engineering, 04.07.2019 19:20
Heat transfer by is the fastest mode of heat transfer that requires no intervening medium. a)-conduction b)-convection c)-radiation d)-conduction and convection
Answers: 1
You know the right answer?
A piston cylinder assembly fitted with a slowly rotating paddle wheel contains 0.13 kg of air at 300...
Questions


Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

Biology, 16.10.2020 17:01



History, 16.10.2020 17:01

History, 16.10.2020 17:01

Arts, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

English, 16.10.2020 17:01

History, 16.10.2020 17:01

Social Studies, 16.10.2020 17:01


Biology, 16.10.2020 17:01