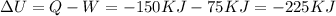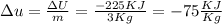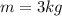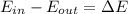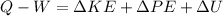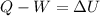Engineering, 13.02.2020 22:47 hockejoh000
A closed system of mass 3 kg undergoes a process in which there is a heat transfer of 150 kJ from the system to the surroundings. The work done on the system is 75 kJ. If the initial specific internal energy of the system is 450 kJ/kg, what is the final specific internal energy, in kJ/kg? Neglect changes in kinetic and potential energy.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
For the closed feedwater heater below, feedwater enters state 3 at a pressure of 2000 psia and temperature of 420 °f at a rate of ix10 ibhr. the feedwat extracted steam enters state 1 at a pressure of 1000 psia and enthalpy of 1500 btu/lbm. the extracted er leaves at an enthalpy of 528.7 btu/lbm steam leaves as a saturated liquid. (16) a) determine the mass flow rate of the extraction steam used to heat the feedwater (10) b) determine the terminal temperature difference of the closed feedwater heater
Answers: 3

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:20
Determine the damped natural frequencies and the steady state response of a decoupled damped forced two degrees of freedom system. 10ä1 + 2q1 20q1 10 cos t; 10q2 +4q2 + 40q2 10 cos t
Answers: 3
You know the right answer?
A closed system of mass 3 kg undergoes a process in which there is a heat transfer of 150 kJ from th...
Questions



Mathematics, 01.07.2019 07:00

English, 01.07.2019 07:00


History, 01.07.2019 07:00

Computers and Technology, 01.07.2019 07:00


History, 01.07.2019 07:00


Biology, 01.07.2019 07:00

Health, 01.07.2019 07:00


Mathematics, 01.07.2019 07:00


History, 01.07.2019 07:00

Physics, 01.07.2019 07:00