
Engineering, 19.10.2019 05:30 hamidaakter936848
An fcc iron–carbon alloy initially containing 0.35 wt% c is exposed to an oxygen-rich and virtually carbon-free atmosphere at 1400 k (1127 °c). under these circumstances the carbon diffuses from the alloy and reacts at the surface with the oxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0 wt% c. (this process of carbon depletion is termed decarburization.) at what position will the carbon concentration be 0.15 wt% after a 10-h treatment? the value of d at 1400 k is 6.9 x 10-11 m2 /s. g

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2

Engineering, 04.07.2019 18:20
How much power could a wind turbine produce if it had the following specifications? cp = 0.45 -d=1.2kg/m3 d=50m v 5m/s
Answers: 2

Engineering, 04.07.2019 19:10
What is a monomer? how do they form a ploymer from the view point of chemical bonding?
Answers: 1
You know the right answer?
An fcc iron–carbon alloy initially containing 0.35 wt% c is exposed to an oxygen-rich and virtually...
Questions

Mathematics, 17.12.2020 19:20

Mathematics, 17.12.2020 19:20





Mathematics, 17.12.2020 19:20

Physics, 17.12.2020 19:20


English, 17.12.2020 19:20


Mathematics, 17.12.2020 19:20

History, 17.12.2020 19:20

Mathematics, 17.12.2020 19:20

English, 17.12.2020 19:20





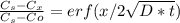
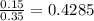
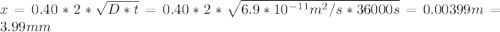 t} )[/tex]
t} )[/tex]

