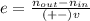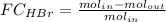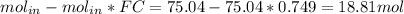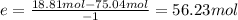Engineering, 16.10.2019 05:00 jnsoccerboy3121
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor. the product stream is analyzed and found to contain 51.7 mole% c2h5br and 17.3% hbr. the feed to the reactor contains only ethylene and hydrogen bromide. calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess. if the molar flow rate of the feed stream is 165mol/s, what is the extent of reaction?

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Apipe with an outside diameter of 15 cm is exposed to an ambient air and surrounding temperature of -20°c. the pipe has an outer surface temperature of 65°c and an emissivity of 0.85. if the rate of heat loss from the pipe surface is 0.95 kw per meter of length, the external convective heat transfer coefficient (h) is: (a) 12.5 w/m"k (b) 18.6 w/mk (c) 23.7 w/mk (d) 27.9 w/mk (e) 33.5 w/mk
Answers: 1

Engineering, 04.07.2019 18:10
Water at 55c flows across a flat plate whose surface temperature is held constant at 95c. if the temperature gradient at the plate's surface for a given value of x is 18 c/mm, find a) local heat transfer coefficient. b) heat flux
Answers: 3

Engineering, 04.07.2019 18:10
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2

You know the right answer?
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continu...
Questions


English, 16.01.2020 03:31

Arts, 16.01.2020 03:31




Mathematics, 16.01.2020 03:31


English, 16.01.2020 03:31





Mathematics, 16.01.2020 03:31

Mathematics, 16.01.2020 03:31

Mathematics, 16.01.2020 03:31

Mathematics, 16.01.2020 03:31



Mathematics, 16.01.2020 03:31


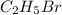 = 51.7%
= 51.7%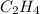 = 100% - 69% = 31%
= 100% - 69% = 31%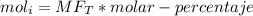
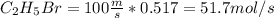
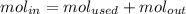
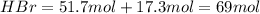
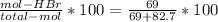 = 45.4%
= 45.4%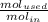
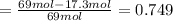
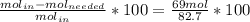 =16.56%
=16.56%