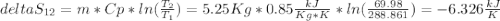Engineering, 11.10.2019 05:30 aidenmanpig
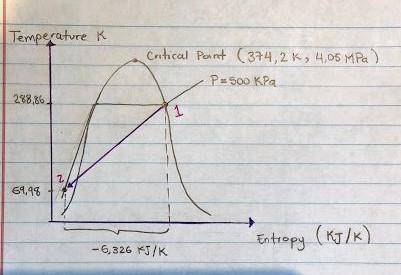
Aweighted, frictionless piston-cylinder device initially contains 5.25 kg of r134a as saturated vapor at 500 kpa. the container loses heat to the surroundings during an isobaric process, the energy lost is 976.71 kj. (a) determine the change in entropy (kj/k) of the r134a during this process. (b) show the process on a t-s diagram

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
Draw the engineering stress-strain curve for (a) bcc; (b) fcc metals and mark important points.
Answers: 1

Engineering, 04.07.2019 19:20
Air at a pressure of 1atm and a temperature of 40 c is in parallel flow over the top surface of a flat plate that is heated to a uniform temperature of 120 c. the plate has a length of 0.40m (in the flow direction) and a width of 0.15m. the reynolds number based on the plate length is 50, 000. what is the rate of heat transfer from the plate to the air? if the free stream velocity of the air is doubled and the pressure is increased to 10 atm what is the rate of heat transfer?
Answers: 2

Engineering, 06.07.2019 02:30
An electric motor is used to drive a power press which makes steel turning moment diagrams 323 pressings from a metal sheet. the motor runs at a mean speed of 50 rev/s. the torque required is 10.0nm for 0.2s, followed by 1.0nm for0.3 s with this sequence then being repeated. what is the minimum power required of the motor and the moment of incrtia required for the t1ywhcel if the speed fluctuations are to be restricted to 1.5%?
Answers: 3
You know the right answer?
Aweighted, frictionless piston-cylinder device initially contains 5.25 kg of r134a as saturated vapo...
Questions



Mathematics, 22.01.2021 05:20


Mathematics, 22.01.2021 05:20

Social Studies, 22.01.2021 05:20


Social Studies, 22.01.2021 05:20

English, 22.01.2021 05:20

English, 22.01.2021 05:20


Chemistry, 22.01.2021 05:20



Mathematics, 22.01.2021 05:20


Mathematics, 22.01.2021 05:20


Mathematics, 22.01.2021 05:20

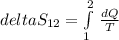
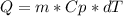

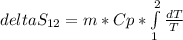
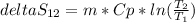
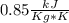
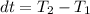 clearing for T2 we get:
clearing for T2 we get: