Engineering, 27.09.2019 23:30 jak000067oyyfia
Aclosed, rigid tank is filled with a gas modeled as an ideal gas, initially at 27°c and a gage pressure of 300 kpa. if the gas is heated to 77°c, determine the final pressure, expressed as a gage pressure, in kpa. the local atmospheric pressure is 1 atm.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 03.07.2019 23:20
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3
You know the right answer?
Aclosed, rigid tank is filled with a gas modeled as an ideal gas, initially at 27°c and a gage press...
Questions

Health, 29.09.2020 14:01

Biology, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01

English, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

English, 29.09.2020 14:01

Biology, 29.09.2020 14:01



English, 29.09.2020 14:01





Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01



History, 29.09.2020 14:01

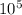 Pa
Pa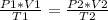 ................1
................1