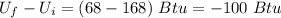Engineering, 13.09.2019 22:30 Yailynn598
The initial internal energy of a mug of coffee is known to be 168 btu. the initial coffee temperature is approximately 200 f. the room temperature is 70 f. time passes and the final amount of internal energy in the coffee is 68 btu. how much heat flowed from the coffee to the room?

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 18:10
Slip occurs via two partial dislocations because of (a) the shorter path of the partial dislocation lines; (b) the lower energy state through partial dislocations; (c) the charge balance.
Answers: 1

Engineering, 04.07.2019 18:10
What are the two (02) benefits, which may result from a successful implementation of preventive maintenance (pm) program in an organization? (clo3)a)- lean manufacturing b)-overlapping responsibilities c)-the planner is not qualified d)-accurate contractor information e)-reduction in equipment redundancies f)-accurate stores information
Answers: 3
You know the right answer?
The initial internal energy of a mug of coffee is known to be 168 btu. the initial coffee temperatur...
Questions

Computers and Technology, 19.10.2019 21:30


History, 19.10.2019 21:30

Mathematics, 19.10.2019 21:30

Biology, 19.10.2019 21:30


Mathematics, 19.10.2019 21:30





Mathematics, 19.10.2019 21:30

Mathematics, 19.10.2019 21:30






Biology, 19.10.2019 21:30

Chemistry, 19.10.2019 21:30