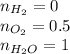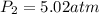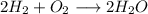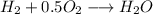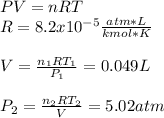Engineering, 02.09.2019 16:30 ineedhelp368
Amixture of 1 kmol of hydrogen (h2) and n kmol of oxygen (o2), initially at 25°c and 1 atm, burns completely in a closed, rigid, insulated container. the container finally holds a mixture of water vapor and o2 at 2000 k. the ideal gas model applies to each mixture and there is no change in kinetic or potential energy between the initial and final states. determine: (a) the value of n. (b) the final pressure, in atm.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
If a particle moves along a path such that r : (3 sin t) m and ? : 2t rad, where t is in seconds. what is the particle's acceleration in m/s in 4 seconds? a)- 16.43 b)- 16.29 c)- 15.21 d)- 13.79
Answers: 1

Engineering, 04.07.2019 18:10
Awall of 0.5m thickness is to be constructed from a material which has average thermal conductivity of 1.4 w/mk. the wall is to be insulated with a material having an average thermal conductivity of 0.35 w/mk so that heat loss per square meter shall not exceed 1450 w. assume inner wall surface temperature of 1200°c and outer surface temperature of the insulation to be 15°c. calculate the thickness of insulation required.
Answers: 3

Engineering, 04.07.2019 19:10
In general, how do thermosetting plastics compare to thermoplastics in mechanical and physical properties?
Answers: 3
You know the right answer?
Amixture of 1 kmol of hydrogen (h2) and n kmol of oxygen (o2), initially at 25°c and 1 atm, burns co...
Questions



Computers and Technology, 23.09.2020 09:01



Computers and Technology, 23.09.2020 09:01


Mathematics, 23.09.2020 09:01


Biology, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01




Mathematics, 23.09.2020 09:01



Health, 23.09.2020 09:01

Chemistry, 23.09.2020 09:01