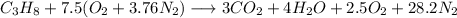Engineering, 29.08.2019 18:00 lsrgb
Propane (c3h8) burns completely with 150% of theoretical air entering at 74°f, 1 atm, 50% relative humidity. the dry air component can be modeled as 21% o2 and 79% n2 on a molar basis. the combustion products leave at 1 atm. determine the mole fraction of water in the products, in lbmol(water)/lbmol(products).

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
Line joining liquid phase with liquid and solid phase mixture is known as: a) liquidus b) solidus c) tie line d) none of the mentioned
Answers: 2


Engineering, 04.07.2019 19:10
What are the major differences between injection molding and extrusion?
Answers: 2

Engineering, 04.07.2019 19:10
What is the major difference between thermoplastics and thermosetting plastics from the polymerization structure point of view?
Answers: 2
You know the right answer?
Propane (c3h8) burns completely with 150% of theoretical air entering at 74°f, 1 atm, 50% relative h...
Questions


Mathematics, 03.10.2021 04:10


Social Studies, 03.10.2021 04:10

Mathematics, 03.10.2021 04:10


Mathematics, 03.10.2021 04:10

Mathematics, 03.10.2021 04:10






History, 03.10.2021 04:10

English, 03.10.2021 04:10


Mathematics, 03.10.2021 04:10

Mathematics, 03.10.2021 04:10

History, 03.10.2021 04:10