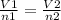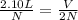Chemistry, 21.07.2019 09:00 itziabejar
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final volume of the gas in liters? (assume the pressure and temperature remain constant.) none of the above 8.40 4.20 1.05 6.30

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final...
Questions


English, 08.11.2019 13:31


Mathematics, 08.11.2019 13:31

English, 08.11.2019 13:31

Mathematics, 08.11.2019 13:31

Computers and Technology, 08.11.2019 13:31

Mathematics, 08.11.2019 13:31

Computers and Technology, 08.11.2019 13:31



Mathematics, 08.11.2019 13:31

Arts, 08.11.2019 13:31


Arts, 08.11.2019 13:31


Mathematics, 08.11.2019 13:31

Mathematics, 08.11.2019 13:31

Mathematics, 08.11.2019 13:31