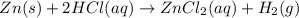Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Given the reaction: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) the oxidation number of zn(s) increases be...
Questions

Mathematics, 11.05.2021 23:40


Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40


Mathematics, 11.05.2021 23:40




Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40

Mathematics, 11.05.2021 23:40





English, 11.05.2021 23:40