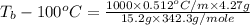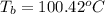Chemistry, 21.07.2019 11:00 joanasprinkman2262
If 4.27 g sucrose (c12h22o11) are dissolved in 15.2 g water, what is the boiling point of the resulting solution? kb for water = 0.512c/m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
If 4.27 g sucrose (c12h22o11) are dissolved in 15.2 g water, what is the boiling point of the result...
Questions

English, 29.04.2021 18:00


Mathematics, 29.04.2021 18:00



Mathematics, 29.04.2021 18:00

Mathematics, 29.04.2021 18:00



Mathematics, 29.04.2021 18:00


Arts, 29.04.2021 18:00


Mathematics, 29.04.2021 18:00



Mathematics, 29.04.2021 18:00

Biology, 29.04.2021 18:00


Biology, 29.04.2021 18:00

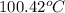
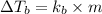
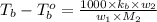
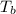 = boiling point of solution = ?
= boiling point of solution = ?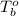 = boiling point of pure water =
= boiling point of pure water = 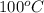
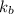 = boiling point constant for water =
= boiling point constant for water = 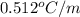
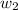 = mass of solute (sucrose) = 4.27 g
= mass of solute (sucrose) = 4.27 g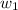 = mass of solvent (water) = 15.2 g
= mass of solvent (water) = 15.2 g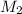 = molar mass of solute (sucrose) = 342.3 g/mole
= molar mass of solute (sucrose) = 342.3 g/mole