
Chemistry, 21.07.2019 15:00 Dmoney7784
Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with 4.000 moles of o2. 2 c8h18(l) + 25 o2(g) → 16 co2(g) + 18 h2o(g) molar mass co2 = 44.01 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

You know the right answer?
Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with...
Questions





Mathematics, 06.01.2020 04:31

Social Studies, 06.01.2020 04:31

Health, 06.01.2020 04:31


Mathematics, 06.01.2020 04:31

Mathematics, 06.01.2020 04:31

Mathematics, 06.01.2020 04:31


Health, 06.01.2020 04:31







History, 06.01.2020 04:31

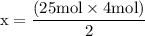 x = 50 moles of oxygen or O₂.
x = 50 moles of oxygen or O₂. 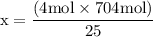 x = 112.64 gram of carbon dioxide
x = 112.64 gram of carbon dioxide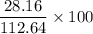 Percentage yield = 25%
Percentage yield = 25%

