
Chemistry, 21.07.2019 23:00 trinidymwilga
In a future hydrogen-fuel economy, the cheapest source of h2 will certainly be water. it takes 467 kj to produce 1 mol of h atoms from water. what is the frequency, wavelength, and minimum energy of a photon that can free an h atom from water? enter your answers in scientific notation.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
In a future hydrogen-fuel economy, the cheapest source of h2 will certainly be water. it takes 467 k...
Questions

Mathematics, 03.12.2020 16:50



Mathematics, 03.12.2020 16:50


Mathematics, 03.12.2020 16:50








Biology, 03.12.2020 16:50

Biology, 03.12.2020 16:50





History, 03.12.2020 16:50

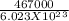 = 7.7536 x 10^(-19) j
= 7.7536 x 10^(-19) j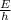 =
= 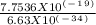 = 1.1702 X 10^(15) Hz
= 1.1702 X 10^(15) Hz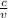
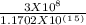
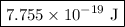
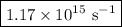 .
.
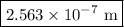 .
.
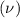 is defined as number of times n event occurs in unit time. It is generally applied to waves including light, sound, and radio waves. It is denoted by
is defined as number of times n event occurs in unit time. It is generally applied to waves including light, sound, and radio waves. It is denoted by  and its SI unit is Hertz (Hz).
and its SI unit is Hertz (Hz).
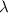 and its SI unit is meter (m).
and its SI unit is meter (m).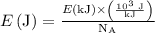 …… (1)
…… (1)
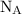 is an Avogadro's number and has a value
is an Avogadro's number and has a value 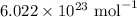 .
.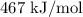 for
for 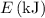 and
and 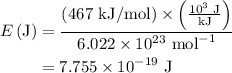
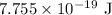 .
.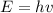 …… (2)
…… (2)
 is a frequency of photon and h is a Plank’s constant and has a value
is a frequency of photon and h is a Plank’s constant and has a value 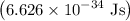 .
.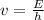 …… (3)
…… (3)
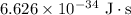 for h and
for h and 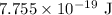 for E in equation (3).
for E in equation (3).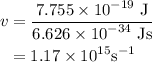
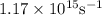 .
.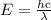 …… (4)
…… (4)
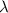 is a wavelength of a photon and c is a speed of light.
is a wavelength of a photon and c is a speed of light.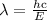 …… (5)
…… (5)
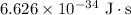 for h,
for h, 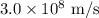 for c and
for c and 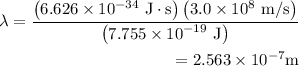
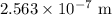 .
.

