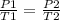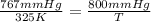Chemistry, 22.07.2019 02:30 bjpvrpow74wq
When the pressure that a gas exerts on a sealed container, with a temperature of 325 k, changes from 767 mm hg to 800 mm hg, what will the new temperature be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1
You know the right answer?
When the pressure that a gas exerts on a sealed container, with a temperature of 325 k, changes from...
Questions

Chemistry, 05.10.2020 14:01


Physics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01




Mathematics, 05.10.2020 14:01

History, 05.10.2020 14:01


History, 05.10.2020 14:01

History, 05.10.2020 14:01

Physics, 05.10.2020 14:01



Social Studies, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01