
Chemistry, 22.07.2019 02:30 Goldenstate32
Which of the following changes occurs with the greatest increase in entropy? (2 points) c(s) + co2 (g) yields 2co (g) 3fe (s) + 2o2 (g) yields fe3o4 (s) 3o2 (g) + 2kcl (s) yields 2kclo3 (s) nh(g) + hcl (g) yields nh4cl (s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 12:30
How do you interpret a chromatogram for what mixtures contain?
Answers: 3

You know the right answer?
Which of the following changes occurs with the greatest increase in entropy? (2 points) c(s) + co2...
Questions


History, 24.09.2019 08:20

Biology, 24.09.2019 08:20

Computers and Technology, 24.09.2019 08:20

History, 24.09.2019 08:20

Mathematics, 24.09.2019 08:20






Mathematics, 24.09.2019 08:20

Health, 24.09.2019 08:20

Mathematics, 24.09.2019 08:20



Mathematics, 24.09.2019 08:20

History, 24.09.2019 08:20


History, 24.09.2019 08:20

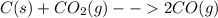 : In this reaction, the number of moles of gaseous products (2 mol) is greater than the number of moles of gaseous reactants (1 mol). Therefore, this reaction proceeds with an increase in entropy.
: In this reaction, the number of moles of gaseous products (2 mol) is greater than the number of moles of gaseous reactants (1 mol). Therefore, this reaction proceeds with an increase in entropy.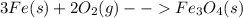 : In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (2 mol). This proceeds with a decrease in entropy.
: In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (2 mol). This proceeds with a decrease in entropy.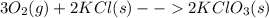 : In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (3 mol). This proceeds with a decrease in entropy.
: In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (3 mol). This proceeds with a decrease in entropy.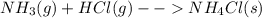 :In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (2 mol). This proceeds with a decrease in entropy.
:In this reaction, the number of moles of gaseous products ( no gases on product side) is less than the number of moles of gaseous reactants (2 mol). This proceeds with a decrease in entropy.

