Chemistry, 22.07.2019 11:30 RickandMorty420710
Consider the reaction of zn metal with hydrochloric acid: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) if 2.57 g of zn is reacted with 0.500 moles of hcl in a 3.00 l container what pressure does the generated h2 exert against the container walls at 35.8 ℃?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
Consider the reaction of zn metal with hydrochloric acid: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) if 2...
Questions

Chemistry, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Medicine, 10.03.2021 01:00

History, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00




Social Studies, 10.03.2021 01:00

Health, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


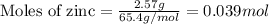
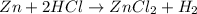
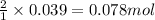 of HCl
of HCl of hydrogen gas
of hydrogen gas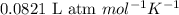
![35.8^oC=[35.8+273]K=308.8K](/tpl/images/0119/3926/fdfeb.png)