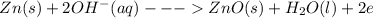Chemistry, 22.07.2019 14:30 justttlearnnn12333
Here is the discharge reaction for an alkaline battery: zn(s) + 2mno2(s) + h2o(l)\longrightarrow⟶⟶zn(oh)2(s) + mn2o3(s) which species is reduced as the battery is discharged?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Here is the discharge reaction for an alkaline battery: zn(s) + 2mno2(s) + h2o(l)\longrightarrow⟶⟶z...
Questions


Business, 02.10.2019 16:30








Geography, 02.10.2019 16:30


Mathematics, 02.10.2019 16:30

Biology, 02.10.2019 16:30

Mathematics, 02.10.2019 16:30





Mathematics, 02.10.2019 16:30