
Chemistry, 22.07.2019 14:30 b2cutie456
Identify the balanced single displacement reaction. cucl2 + fe ⟶ 2cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cacl2 + pb(oh)4 cucl2 + fe ⟶ cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cacl2 + 4pb(oh)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
Identify the balanced single displacement reaction. cucl2 + fe ⟶ 2cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cac...
Questions


Physics, 21.07.2019 02:50


Mathematics, 21.07.2019 03:00




Chemistry, 21.07.2019 03:00

English, 21.07.2019 03:00


Social Studies, 21.07.2019 03:00


Mathematics, 21.07.2019 03:00

Mathematics, 21.07.2019 03:00


Computers and Technology, 21.07.2019 03:00

History, 21.07.2019 03:00



Mathematics, 21.07.2019 03:00

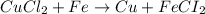
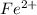 in
in 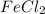 and
and 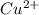 in
in 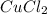 accepts electrons to form
accepts electrons to form 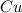 .
.
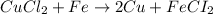 : The equation is not balanced.
: The equation is not balanced.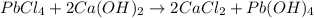 : This is a double displacement reaction and is balanced.
: This is a double displacement reaction and is balanced.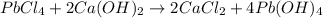 : This is a double displacement reaction and is not balanced.
: This is a double displacement reaction and is not balanced.

