
Chemistry, 22.07.2019 18:30 jesh0975556
In the lab, frank has two solutions that contain alcohol and is mixing them with each other. he uses 100 milliliters less of solution a than solution b. solution a is 13% alcohol and solution b is 17% alcohol. how many milliliters of solution b does he use, if the resulting mixture has 347 milliliters of pure alcohol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
In the lab, frank has two solutions that contain alcohol and is mixing them with each other. he uses...
Questions

Mathematics, 04.01.2021 15:10

English, 04.01.2021 15:10

History, 04.01.2021 15:10

English, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10


Social Studies, 04.01.2021 15:10

Chemistry, 04.01.2021 15:10

History, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

Business, 04.01.2021 15:10

Geography, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

English, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

Biology, 04.01.2021 15:10

History, 04.01.2021 15:10

Mathematics, 04.01.2021 15:10

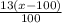 = 0.13(x-100),
= 0.13(x-100),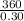 = 1200
= 1200

