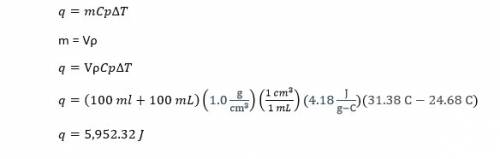In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both solutions were originally at 24.68c. after the reaction, the final temperature is 31.38c. assuming that all the solutions have a density of 1.0 g/cm3 and a specific heat capacity of 4.18 j/8c ? g, calculate the enthalpy change for the neutralization of hcl by naoh

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both soluti...
Questions

History, 07.09.2020 02:01


Mathematics, 07.09.2020 02:01


History, 07.09.2020 02:01


Computers and Technology, 07.09.2020 02:01






English, 07.09.2020 02:01



Mathematics, 07.09.2020 02:01

Mathematics, 07.09.2020 02:01



Mathematics, 07.09.2020 02:01