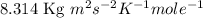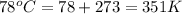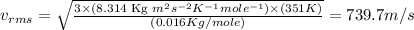Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
Calculate the root-mean-square speed of methane, ch4 (g), at 78°c. root mean square speed = u = [3rt...
Questions


World Languages, 28.07.2019 08:30



Mathematics, 28.07.2019 08:30



History, 28.07.2019 08:30





Mathematics, 28.07.2019 08:30



History, 28.07.2019 08:30




Mathematics, 28.07.2019 08:30

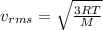
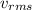 = root mean square speed
= root mean square speed