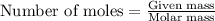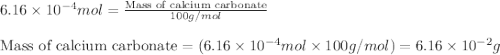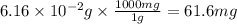Chemistry, 23.07.2019 10:00 coralstoner6793
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s) + 2h+(aq) > ca(2+)(aq) + co2(g) + h2o (l) 15.5ml of co2 was produced at 25*c and 738.0 mmhg how man moles of co2 were produced? how many milligrams of caco3 were consumed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s...
Questions


English, 05.12.2019 04:31




History, 05.12.2019 04:31


History, 05.12.2019 04:31


Mathematics, 05.12.2019 04:31



Biology, 05.12.2019 04:31



English, 05.12.2019 04:31

English, 05.12.2019 04:31


Biology, 05.12.2019 04:31

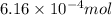 and the mass of calcium carbonate is 61.6 mg
and the mass of calcium carbonate is 61.6 mg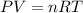
![25^oC=[25+273]K=298K](/tpl/images/0123/0054/df1f6.png)
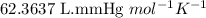

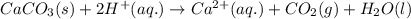
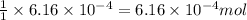 of calcium carbonate
of calcium carbonate