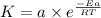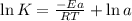Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
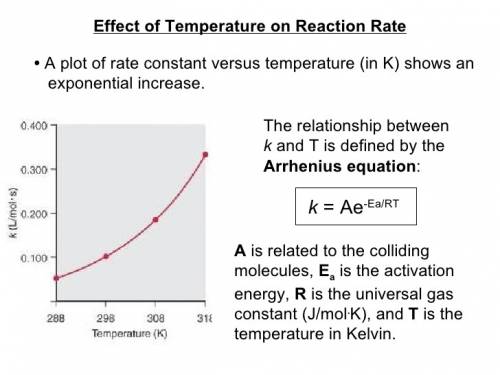
The first order reaction of methyl isonitrile to form acetonitrile has a rate constant of k = 1.7 ×...
Questions



Social Studies, 24.07.2019 13:20


History, 24.07.2019 13:20



History, 24.07.2019 13:20

History, 24.07.2019 13:20

Biology, 24.07.2019 13:20


Advanced Placement (AP), 24.07.2019 13:20

Chemistry, 24.07.2019 13:20

History, 24.07.2019 13:20



Health, 24.07.2019 13:20

Health, 24.07.2019 13:20