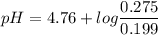Chemistry, 23.07.2019 12:30 teesoprettyy
Calculate the ph of 0.375 l of a 0.18 m acetic acid-0.29 m sodium acetate buffer after the addition of 0.0070 mol of hbr. assume that the volume remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

You know the right answer?
Calculate the ph of 0.375 l of a 0.18 m acetic acid-0.29 m sodium acetate buffer after the addition...
Questions


Mathematics, 22.07.2019 10:30




Mathematics, 22.07.2019 10:31

History, 22.07.2019 10:31

Mathematics, 22.07.2019 10:31

Mathematics, 22.07.2019 10:31

Mathematics, 22.07.2019 10:31

English, 22.07.2019 10:31




Mathematics, 22.07.2019 10:31


Advanced Placement (AP), 22.07.2019 10:31


Biology, 22.07.2019 10:31

Mathematics, 22.07.2019 10:31

![\displaystyle [H^+]=Ka\times\frac{mole\:weak\:acid}{mole\:salt\times valence}](/tpl/images/0123/4241/7d032.png)
![\displaystyle [OH^-]=Kb\times\frac{mole\:weak\:base}{mole\:salt\times valence}](/tpl/images/0123/4241/6b85e.png)
![\displaystyle pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0123/4241/3aa97.png)