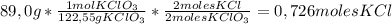Chemistry, 23.07.2019 15:00 monaae3824
How many moles of kcl will be produced from 89.0 g of kclo3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
How many moles of kcl will be produced from 89.0 g of kclo3...
Questions


Mathematics, 22.03.2021 22:20

Mathematics, 22.03.2021 22:20

Arts, 22.03.2021 22:20

Biology, 22.03.2021 22:20

Mathematics, 22.03.2021 22:20

Mathematics, 22.03.2021 22:20





Biology, 22.03.2021 22:20


Mathematics, 22.03.2021 22:20



Mathematics, 22.03.2021 22:20


History, 22.03.2021 22:20