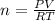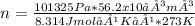Chemistry, 23.07.2019 17:00 elliedeegan3910
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mass in the sample. for the above problem how will you rearrange the ideal gas law to solve for moles of argon?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mas...
Questions



Computers and Technology, 10.03.2020 00:22



Computers and Technology, 10.03.2020 00:22











Computers and Technology, 10.03.2020 00:22