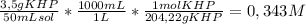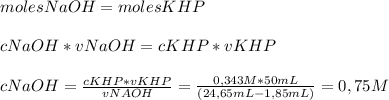To determine the molarity of naoh solution, student took 3.5 g of khp (khp – potassium hydrogen phthalate; molar mass = 204.22 g/mol) and dissolved in 50 ml of water and titrated with the given unknown molarity naoh solution loaded in burette. his burette volume read 1.85 ml at the start of the experiment and 24.65 ml when the phenolphthalein indicator turned pink. using above experimental details, determine the molarity of naoh solution? (enter your answer in two decimal palaces).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

You know the right answer?
To determine the molarity of naoh solution, student took 3.5 g of khp (khp – potassium hydrogen phth...
Questions


Social Studies, 16.04.2020 21:09

Mathematics, 16.04.2020 21:09



Mathematics, 16.04.2020 21:09



Mathematics, 16.04.2020 21:09



Mathematics, 16.04.2020 21:09

English, 16.04.2020 21:09

Biology, 16.04.2020 21:09





Advanced Placement (AP), 16.04.2020 21:09