
Chemistry, 23.07.2019 23:30 zarrialamons16
Which represents the self-ionization of water at 25°c? h2o + h2o 2h2 + o2 h2o + h2o h2o2 + h2 h2o + h2o 4h+ + 2o2- h2o + h2o h3o+ + oh-

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

You know the right answer?
Which represents the self-ionization of water at 25°c? h2o + h2o 2h2 + o2 h2o + h2o h2o2 + h2 h2o +...
Questions







Business, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43


Social Studies, 28.10.2019 00:43



Biology, 28.10.2019 00:43

Mathematics, 28.10.2019 00:43

History, 28.10.2019 00:43


Chemistry, 28.10.2019 00:43


English, 28.10.2019 00:43

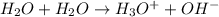
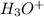 ) and hydroxide ion (
) and hydroxide ion (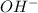 ).
).

