Chemistry, 24.07.2019 06:00 lilque6112
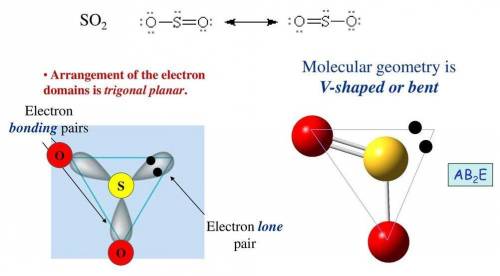
Etermine the electron geometry (eg), molecular geometry (mg), and polarity of so2. determine the electron geometry (eg), molecular geometry (mg), and polarity of . eg = tetrahedral, mg = tetrahedral, nonpolar eg = trigonal pyramidal, mg = trigonal pyramidal, polar eg = linear, mg = bent, nonpolar eg = tetrahedral, mg = linear, nonpolar eg = trigonal planar, mg = bent, polar

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Etermine the electron geometry (eg), molecular geometry (mg), and polarity of so2. determine the ele...
Questions

Mathematics, 09.03.2022 18:20


Mathematics, 09.03.2022 18:30


Mathematics, 09.03.2022 18:30


English, 09.03.2022 18:30



Geography, 09.03.2022 18:30

Mathematics, 09.03.2022 18:30


Mathematics, 09.03.2022 18:30


Mathematics, 09.03.2022 18:30

English, 09.03.2022 18:30

Computers and Technology, 09.03.2022 18:40

English, 09.03.2022 18:40


Mathematics, 09.03.2022 18:40