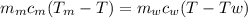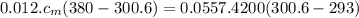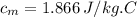Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Apiece of metal of mass 12 g at 107 ◦c is placed in a calorimeter containing 55.7 g of water at 20◦c...
Questions





Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01






English, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01



Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01